Does the use of menstrual cups increase the risk of toxic shock syndrome?
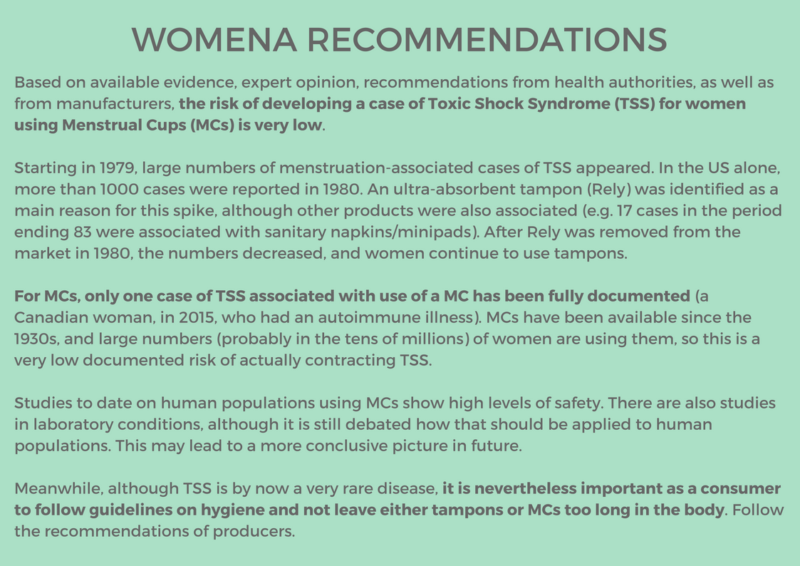
QUESTION: Does use of menstrual cups increase the risk of toxic shock syndrome (tss)?
TSS – WHAT IS IT?
TSS is an autoimmune reaction by the body to toxins produced by the bacteria Staphylococcus aureus or Streptococcus pyogenes. S. pyogenes is associated with more generalised infections, while S. aureus is the bacterium most commonly related to menstruation-associated TSS, which we will refer to as mTSS[1].
- aureus and TSS toxins are commonly present in body cavities such as the vagina or nasal cavities without causing harm. However, if bacterial growth increases rapidly, causing great amounts of toxins to be produced, and there is a way for the toxin to enter the blood stream, this can cause the autoimmune reaction. deVries notes that S. aureus toxins called superantigens, in combination with susceptibility of the host (due to absence of the anti-superantigen antibodies) are key factors for developing TSS ((DeVries, et al. 2011).
Symptoms for diagnosis (case definition) are generally described as including: high fever (above 38.9 C), low blood pressure (<90 systolic mmHg), sore throat, vomiting, diarrhoea, confusion, muscle/headache, malaise, red and peeling skin (after 1-2 weeks) (CDC ; Lægehåndbogen)
This clinical syndrome (set of symptoms) has been described sporadically since the 1920’s (Hajjeh, et al. 1999). The term ‘TSS’ was first coined in 1978, by an American medical doctor reporting on seven boys and girls aged eight to 17 years with an acute febrile illness (Todd, et al. 1978).
TSS is coded by the WHO as ICD-10- A48.3.
WHO IS AT RISK FOR GETTING mTSS?
In 1980, public attention was drawn to a large number of cases of TSS in young menstruating women using tampons, especially a brand called ‘Rely’, which was introduced in the US in 1975 and actively marketed in 1978 (Hajjeh, et al. 1999; Reingold, et al. 1989). Suggested risk factors included prolonged continuous use (allowing for growth of bacteria), and also that the tampons were ultra- absorbent, causing expansion of the tampon and vaginal dryness, which in turn caused abrasions (lesions) when tampons were removed (Vostral 2011). Rely was removed from the market shortly thereafter. Nevertheless, this heightened the awareness that there might be an association between menstruation and TSS.
Since this occurrence in 1980, the US Centers for Disease Prevention and Control (CDC) has reported on TSS, whatever the cause and population concerned (CDC). Hajjeh et al note that overall surveillance seems fairly complete. The surveillance includes details such as menstrual product used, although apparently it is not always reported. An update from the CDC in 1983 notes that, out of 1535 cases of mTSS where product type was reported in the period ending 1983, 1517 were using tampons, 17 were using napkins/minipads, and 1 was using a sea sponge (CDC 1983). There is required reporting on any adverse effect of medical devices to the Federal Drug Administration (MAUDE), but here questions are raised about completeness and accuracy. There has been a great increase in studies since 1980, and this has contributed to a better understanding of both levels and possible causes (CDC), although much of it comes from US populations.
Many behavioural risk factors have been considered or identified for both for mTSS. In 1983 there were 4 reported cases of TSS among U.S. women using the ‘Today’ contraceptive sponge, but no recommendation was made to withdraw the sponge from the market (CDC 1984). Other possible cases have been associated with IUD use, but not confirmed (CDC-MAUDE). There are also concerns about other high absorbency products such as high-absorbency diapers (Hajjeh, et al. 1999).
HOW COMMON IS IT?
TSS is very rare, and the incidence has decreased since 1980.
The incidence of TSS in 1980 among young menstruating women in the US was estimated at 13.7 per 100,000, but by 1986 it had fallen to 1 per 100,000 for menstrual, and 0.3 for non-menstrual cases (DeVries, et al. 2011). An active surveillance study in Minnesota in the US, for the period 2000-2006, found an average annual incidence per 100,000 of 0.69/100,000 for menstrual, and 0.32/100,000 for non-menstrual cases (DeVries, et al. 2011).
The CDC attributes the spike in 1980, and subsequent drop, to the removal of Rely, but also to better labelling, behavioural change and removal of some components of Rely which made it super-absorbent, such as polyacryl (Hajjeh, et al. 1999).
We have not been able to find data from Denmark or Uganda which are easily comparable, for example the incidence of TSS related to S. pyogenes was 2.6 per 100,000 in the period 2003-4 (Luca-Harari, et al. 2008), but for S. aureus the estimates seem to refer to the deVries study (Lægehåndbogen). This may be due to differences in reporting systems and metrics, but all accessed sources indicate a very low incidence.
HOW DANGEROUS IS IT?
The mortality among people diagnosed with TSS varies. The CDC surveillance data suggest that case fatality for mTSS was lower than for other cases (Hajjeh 1999). This is confirmed by a study from France which found that there were 55 cases of reported TSS in France during 31 months in 2003-2006, with 0% mortality for the 21 cases of menstruating women, and 22% in the other cases (Descloux, et al. 2008).
IS MC USE ASSOCIATED WITH REPORTED CASES OF TSS?
There has been only one case of TSS associated with MC use, in published peer-reviewed literature, and living up to CDC criteria for diagnosis. It occurred in a 37-year-old Canadian woman, who reported this was the first time she used a MC (Diva), and that insertion caused an abrasion. The woman suffered from Hashimoto’s thyroiditis (an autoimmune condition) and chronic menorrhagia (heavy menstrual bleeding) (Mitchell et al., 2015).
The US Federal Drug Administration (FDA) reported a case from 2008 in a woman who was using an MC, although association was not finally established (CDC-MAUDE). TSS has also been reported in a woman who was using both an IUD and a MC. There was no conclusion as to whether the IUD or the MC was responsible.
The above-mentioned study by Descloux found that 19 out of 21 cases of mTSS reported using tampons. No indication is given for the product for the remaining two.
To establish the risk, one has to know both how many cases there are, as well as how many women are using the MC. To establish relative risk one has to estimate that same risk for all alternative products.
We have not been able to establish a definite prevalence of use of any menstrual product.
For MCs, a rough estimate, based on sales figures and market analyses, is that several million are purchased every year, although there are indications it may be in the tens of millions. Given that MCs are used for up to 10 years, the number of users is presumably higher (WoMena 2018). A study by North et al (North and Oldham 2011) refers to post-marketing surveillance of 100 million Softcups, which has been conducted by the manufacturer and by the FDA Medwatch system, however, these are disposable and not necessarily comparable.
Given that there are at most a couple of cases which suggest that MC use was associated with an actual TSS case, the risk seems very low indeed, and lower than other products such as tampons, which nevertheless remain in popular use.
IS MCs USE ASSOCIATED WITH DIRECT OR INDIRECT RISK FACTORS?
In addition to actual cases of TSS, several studies have looked at possible risk preconditions for TSS. The necessary preconditions for mTSS are a rapid growth of S. aureus bacteria, production of toxins, entry into the bloodstream and a bodily immune response.. Therefore, researchers have tried to measure increases in bacteria and toxins, either in the body or under laboratory conditions.
A clinical study to examine bacterial growth and toxin in various types of tampons, menstrual cups and diaphragms concluded that cotton tampons have a lower risk than hyper-absorbent synthetic brands. It found no evidence of toxin in the MC tested (Tassaway) (Tierno 1994)
A clinical study of 406 US women through 3 months of MC use found no increase in S. aureus, toxicity or mutagenicity. The conclusion was that ‘the single-size vaginal device has no significant health risk and is acceptable to many women without need for fitting or other medical services’ (North and Oldham 2011). However, these were disposable MCs ad maybe not comparable.
Studies are beginning to become available outside the US. A feasibility study involving 604 girls in Kenya performed laboratory tests for the girls before and after introduction of pads or MCs. It found no evidence to indicate that use of MCs increases production of S. aureus or TSS toxins (Juma, et al. 2017). Another study from Kenya of 751 school girls found that ‘Provision of menstrual cups and sanitary pads for approximately 1 school-year was associated with a lower STI risk, and cups with a lower bacterial vaginosis risk’ than those who did not receive free products (Phillips-Howard, et al. 2016 ). Both studies conclude it would be good to supplement with large-scale trials and post marketing surveillance.
In the summer of 2017, a study by Gérard Lina from the University of Lyon received wide notice in French and British media. Dr. Lina was reported as having done a study where he collected 700 used cups and tampons, and then tested in a laboratory for bacterial growth and toxins. Media headlines reporting on the study were dramatic, for example on 6 July the Metro in the UK reported ‘Menstrual Cups Are More Likely To Cause Toxic Shock Than Tampons, Claims Study’ (Larbi 2017). However, the next day a response by Dr. Lina was quoted in Le Monde: ‘Our laboratory analyses indicate that the tested products, tampons and menstrual cups, are good products. No tested product promotes the growth of staphylococcus and production of toxin’. Dr Lina deplored alarmist conclusions cited by the media, noting that his results were misinterpreted (Charrid 2017). He noted that none of the 700 users in the study actually had TSS (indeed this would not be expected in a population of only 700). Dr. Lina informed WoMena in July 2017 that he had not yet published, and was hoping to publish in a peer reviewed journal (Lina 2017). We understand that study has not yet been published.
A study published 20 April 2018, with G. Lina as senior author (Nonfoux, et al. 2018) was reported on in the media the same day: ‘Menstrual cups may pose greater risk of toxic shock syndrome than tampons, study claims’(Petter 2018). The actual study conclusions are less emphatic: ‘Notably, our results do not show that menstrual cups are safer than tampons and suggest that they require similar precautions’. The study uses a different protocol from that in 2017, testing various brands of tampons or MCs, placed in a bag with a substrate to allow bacterial growth. The authors find that some products which allow for more air in contact with bacteria (whether tampons, including all-cotton, or MCs) are favourable to higher growth of S. aureus. The authors note some study limitations, e.g. that the study design might not entirely have controlled for how much air was introduced during the procedure. They also find that bacteria may adhere to the MC, calling for more thorough cleaning.
As this FAQ was finalised, many questions remain, both regarding study design and what conclusions can be drawn, given that other laboratory studies have had differing results, and that studies on actual human beings also have differing results. It is interesting that such different studies have resulted in such similar headlines.
Even though we could find no evidence that MCs actually have caused TSS, and indeed only limited evidence of increased risk factors such as growth of S. aureus or increase in TSS toxins, there are theories about what theoretically might do so, particularly changes in the vaginal biome.
These theories include that bacteria need a nutrient, oxygen and time to grow, and this is provided by prolonged continuous MC use. Others theorise that bacteria would find the composition or environment of MCs hospitable for growth. Yet others (Mitchell 2015) theorise that pH might rise with MC use, and this would provide a hospitable environment for bacterial growth.
The study by Tierno et al concludes that S. aureus could not grow on the elastomeric polymer used by Tassaway menstrual cups. On the other hand, slightly elevated levels of were found in the contraceptive diaphragms tested, which were made from latex (Tierno and Hanna 1994).
A study by North et al including 406 US women in seven centres using either MCs or pads, and performed clinical testing for a wide range of issues, including Pap smears, colposcopy, urinalysis, vaginal pH, wet mounts, gram stain, and vaginal microflora cultures. There was no increase in risk factors, such as pH (it remained at around 4.5-6 throughout 3 months of use) (North and Oldham 2011).
That is, as far as we can judge, at this point it is clear that TSS levels are low, that there is little or no direct evidence to suggest that use of MCs increases TSS levels (and that they are lower than for some other products), and that evidence on possible risk factors is still being debated.
RECOMMENDATIONS BY HEALTH AUTHORITIES AND MANUFACTURERS
We could find no recommendations by WHO, CDC or national health boards. In Denmark, the Health Board has not made a pronouncement. An oft-referenced gynaecologist (Head of Department at Hvidovre Hospital), Charlotte Wilken-Jensen, has been quoted as saying she has found no cases of TSS associated with use of menstrual cups (Oehlenschläger). In Denmark this is important, as medical doctors have leeway in deciding what to recommend, and refer to authoritative experts in the absence of official instructions. No authors disrecommend using MCs. Several authors suggest that hygienic practices should be used, that cups should not be left in ‘too long’, or, in Dr Lina’s case, that MCs should be cleaned more assiduously than presently recommended. None disrecommend using MCs.
As is often the case, many questions remain.
UPDATING
WoMena stays actively updated and follow any changes in the recommendation from the various health authorities and manufacturers, as well as noting any anecdotal evidence on adverse events which may emerge. Since we are primarily based in Denmark and Uganda, wherever available we refer to recommendations in those countries, as well as from international sources such as WHO, or expert opinion.
[1] Hajjeh et al give the definition of mTSS as : ‘onset of symptoms occurred within 3 days of the beginning or end of menses’(Hajjeh, et al. 1999)
COMMENTS ARE WELCOME BELOW!
REFERENCES:
CDC. Morbidity and Mortality Weekly Report (MMRW). https://www.cdc.gov/mmwr/volumes/65/wr/mm6522md.htm. Accessed 26 April 2018.
CDC. Toxic shock syndrome other than streptococcal case definition https://wwwn.cdc.gov/nndss/conditions/toxic-shock-syndrome-other-than-streptococcal/case-definition/2011/. Accessed 26 April 2018.
CDC. We were there – lecture 19 October 2017. https://www.cdc.gov/od/science/wewerethere/toxicshock/index.html. Accessed
CDC. 1983. Update: Toxic-Shock Syndrome — United States. https://www.cdc.gov/mmwr/preview/mmwrhtml/00000119.htm. Accessed 6 May 2018.
CDC. 1984. Toxic shock syndrome and the vaginal contraceptive sponge. MMWR CDC Surveillance Summaries 33(4).
CDC-MAUDE, (Manufacturer and User Facility Experience Database). https://www.fda.gov/medicaldevices/deviceregulationandguidance/postmarketrequirements/reportingadverseevents/ucm127891.htm. Accessed 26 April 2018.
Charrid, M. 2017. Coupe menstruelle ou tampon aucune protection testée ne favorise la production de toxine. Le Monde Santé. http://www.lemonde.fr/sante/article/2017/07/07/coupe-menstruelle-ou-tampon-aucune-protection-testee-ne-favorise-la-production-de-toxines_5157515_1651302.html#VLpWWTtRRx1B7WQ0.99. Accessed 26 April 2017.
Descloux, E., T. Perpoint, T. Ferry, G. Lina, M. Bes, F. Vandenesch, I. Mohammedi, and J. Etienne. 2008. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis 27(1):37-43. doi:10.1007/s10096-007-0405-2.
DeVries, A. S., L. Lesher, P. M. Schlievert, T. Rogers, L. G. Villaume, R. Danila, and R. Lynfield. 2011. Staphylococcal toxic shock syndrome 2000-2006: epidemiology, clinical features, and molecular characteristics. PLoS One 6(8):e22997. doi:10.1371/journal.pone.0022997.
Hajjeh, R. A., A. Reingold, A. Weil, K. Shutt, A. Schuchat, and B. A. Perkins. 1999. Toxic shock syndrome in the United States: surveillance update, 1979 1996. Emerging Infectious Diseases 5(6):807-10. doi:10.3201/eid0506.990611.
Juma, J., E. Nyothach, K. F. Laserson, C. Oduor, L. Arita, C. Ouma, K. Oruko, J. Omoto, L. Mason, K. T. Alexander, B. Fields, C. Onyango, and P. A. Phillips-Howard. 2017. Examining the safety of menstrual cups among rural primary school girls in western Kenya: observational studies nested in a randomised controlled feasibility study. BMJ Open 7(4):e015429. doi:10.1136/bmjopen-2016-015429.
Larbi, M. 2017. Menstrual cups are more likely to cause toxic shock than tampons claim study. http://metro.co.uk/2017/07/06/menstrual-cups-are-more-likely-to-cause-toxic-shock-syndrome-than-tampons-claims-study-6758784/. Accessed 26 April 2018.
Lina, G. 2017. Personal communication 2017.08.07 with Siri Tellier, WoMema knowledge management team.
Luca-Harari, B., K. Ekelund, M. van der Linden, M. Staum-Kaltoft, A. M. Hammerum, and A. Jasir. 2008. Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J Clin Microbiol 46(1):79-86. doi:10.1128/JCM.01626-07.
Lægehåndbogen. Toksisk shock syndrom. https://www.sundhed.dk/sundhedsfaglig/laegehaandbogen/infektioner/tilstande-og-sygdomme/bakteriesygdomme/toksisk-shock-syndrom-tss/. Accessed
Nonfoux, L., M. Chiaruzzi, C. Badiou, J. Baude, A. Tristan, J. Thioulouse, D. Muller, C. Prigent Combaret, and G. Lina. 2018. Impact of currently marketed tampons and menstrual cups on Staphylococcus aureus growth and TSST-1 production in vitro. Appl Environ Microbiol. doi:10.1128/AEM.00351-18.
North, B. B., and M. J. Oldham. 2011. Preclinical, clinical, and over-the-counter postmarketing experience with a new vaginal cup: menstrual collection. J Womens Health (Larchmt) 20(2):303-11. doi:10.1089/jwh.2009.1929.
Oehlenschläger, E. Menstruationskopper – så geniale at man tror det er løgn. 5 august 2012. Politiken. https://politiken.dk/forbrugogliv/livsstil/art5554286/L%C3%A6ge-Menstruationskopper-er-s%C3%A5-geniale-at-man-tror-det-er-l%C3%B8gn. Accessed 26 April 2018.
Petter, Olivia. 2018. Menstrual cups may pose greater risk of toxic shock syndrome than tampons, study claims.Date:2018.04.20 16:24 BST. Independent. https://www.independent.co.uk/life-style/health-and-families/toxic-shock-syndrome-mooncups-tampons-risk-tss-menstrual-america-study-a8314546.html. Accessed 26 April 2018.
Phillips-Howard, P. A., E. Nyothach, F. O. Ter Kuile, J. Omoto, D. Wang, C. Zeh, C. Onyango, L. Mason, K. T. Alexander, F. O. Odhiambo, A. Eleveld, A. Mohammed, A. M. van Eijk, R. T. Edwards, J. Vulule, B. Faragher, and K. F. Laserson. 2016 Menstrual cups and sanitary pads to reduce school attrition, and sexually transmitted and reproductive tract infections: a cluster randomised controlled feasibility study in rural Western Kenya. BMJ Open 6(11):e013229. doi:10.1136/bmjopen-2016-013229.
Reingold, A. L., C. V. Broome, S. Gaventa, and A. W. Hightower. 1989. Risk factors for menstrual toxic shock syndrome: results of a multistate case-control study. Rev Infect Dis 11 Suppl 1:S35-41; discussion S41-2.
Tierno. 1994. Propensity of Tampons and Barrier Contraceptives to Amplify Staphylococcus aureus Toxic Shock Syndrome Toxin-I.
Tierno, P. M., and B. A. Hanna. 1994. Propensity of tampons and barrier contraceptives to amplify Staphylococcus aureusToxic shock syndrome toxin-I. Infect Dis Obstet Gynecol 2(3):140-5. doi:10.1155/S1064744994000542.
Todd, J., M. Fishaut, F. Kapral, and T. Welch. 1978. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet 2(8100):1116-8.
Vostral, S. L. 2011. Rely and Toxic Shock Syndrome: a technological health crisis. Yale J Biol Med 84(4):447-59.
WoMena. 2018. How many women are using Menstrual Cups? https://womena.dk/news/. Accessed 6 May 2018.

